“From this point forward, President Trump will only accept from drug manufacturers a commitment that provides American families immediate relief from vastly inflated drug prices and an end to the freeriding by European and other developed nations on American innovations.” – White House Fact Sheet on Most Favored Nation Pricing
 Late last week, President Donald Trump sent letters to 17 pharmaceutical companies pressing them to deliver on his May Executive Order 14297, titled “Delivering Most-Favored-Nation Prescription Drug Pricing to American Patients.” Trump gave the companies 60 days to extend Most Favored Nation (MFN) pricing to all Medicaid patients, for newly launched drugs, to return increased revenues from negotiations with countries to American patients, and to provide for direct purchasing options to patients at MFN rates.
Late last week, President Donald Trump sent letters to 17 pharmaceutical companies pressing them to deliver on his May Executive Order 14297, titled “Delivering Most-Favored-Nation Prescription Drug Pricing to American Patients.” Trump gave the companies 60 days to extend Most Favored Nation (MFN) pricing to all Medicaid patients, for newly launched drugs, to return increased revenues from negotiations with countries to American patients, and to provide for direct purchasing options to patients at MFN rates.
Trump’s Executive Order aims to reduce drug prices “almost immediately, by 30% to 80%” via a “most favored nations” policy that will mandate U.S. citizens pay the same price as the nation paying the lowest price. As a result, prices in other developed countries will rise “to equalize,” said Trump in a May social media post.
High drug prices in the United States are often blamed on patents, but many in the IP realm have argued the situation is far more complex. “One of the chief reasons Americans pay more is the existence of middlemen known as pharmacy benefit managers (PBMs), which only exist in the United States,” wrote IPWatchdog CEO and Founder Gene Quinn recently. “These PBMs do nothing to discover or innovate drugs, and likewise do not sell the drugs to consumers, but somehow they take a staggering 50% of the overall cost of pharmaceutical drugs. The other major differentiator is the fact that research and development is paid for almost exclusively by Americans because other rich western countries implement strict price controls and refuse to pay for R&D.”
Industry experts like Sherry Knowles have also repeatedly attempted to provide more clarity on the nuances of the U.S. drug pricing problem (see below, left).
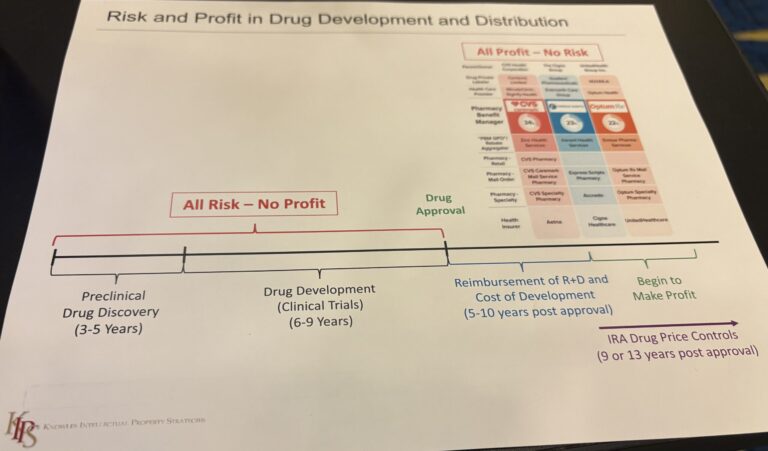 Trump’s Order has been met with skepticism by the industry and others, with Stephen J. Ubl, the president and CEO of PhRMA, saying in statements made to the press in May that “importing foreign prices will cut billions of dollars from Medicare with no guarantee that it helps patients or improves their access to medicines.”
Trump’s Order has been met with skepticism by the industry and others, with Stephen J. Ubl, the president and CEO of PhRMA, saying in statements made to the press in May that “importing foreign prices will cut billions of dollars from Medicare with no guarantee that it helps patients or improves their access to medicines.”
While the U.S. Chamber of Commerce in May agreed with Trump’s view that foreign price controls unfairly shift price burden to the United States, in a statement released Friday the Chamber’s Executive Vice President and Chief Policy Officer Neil Bradley said: “Importing foreign price controls into the U.S. healthcare system will harm patients by delaying access to new, life-saving medicines…. [O]ur research highlights that such policies could lead to a 75% decline in U.S. clinical trial activity, jeopardizing innovation in critical areas like cancer, obesity, and rare and chronic diseases.”
Patient advocacy groups have met Trump’s Order with cautious optimism, with Patients for Affordable Drugs Now issuing a statement on Friday that thanked Trump for making it “clear that the administration recognizes that drug companies are gaming the system to keep prices high here in the U.S.,” but adding that “the letters still leave far too much room for the industry to protect its profits while also raising prices for patients abroad.” The statement raised a number of questions about how the letter’s mandates would be enforced in practice, such as “how would the policy work when Medicaid’s Best Price is already lower than the MFN price?”

Source: @LuxAlgo
Thursday’s letters threatened pharmaceutical companies with consequences if they “refuse to step up.” According to a fact sheet published last week, if the companies don’t respond, “the federal government ‘will deploy every tool in our arsenal to protect American families from continued abusive drug pricing practices.’”
The fact sheet added that the companies have “fallen short” since the Executive Order was first published and that the letters are meant to put further pressure on the industry. “From this point forward, President Trump will only accept from drug manufacturers a commitment that provides American families immediate relief from vastly inflated drug prices and an end to the freeriding by European and other developed nations on American innovations,” said the Fact Sheet.
Trump was quoted as saying that American citizens are “effectively subsidizing socialism aboard [abroad] with skyrocketing prices at home. So we would spend tremendous amounts of money in order to provide inexpensive drugs to another country. And when I say the price is different, you can see some examples where the price is beyond anything — four times, five times different.”
But some commentators have called Trump’s pricing proposals “mathematically impossible.”
The letters were sent to the CEOs of Novo Nordisk, Eli Lilly, GlaxoSmithKline, Abbvie and Pfizer, Amgen, AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, EMD Serono, Genentech, Gilead, Johnson & Johnson, Merck, Novartis, Regeneron and Sanofi.
Note: This article was updated on August 4 to include a more recent statement from the U.S.Chamber of Commerce.

![[IPWatchdog Logo]](https://ipwatchdog.com/wp-content/themes/IPWatchdog%20-%202023/assets/images/temp/logo-small@2x.png)

![[Advertisement]](https://ipwatchdog.com/wp-content/uploads/2026/02/Junior-AI-Feb-10-2026-sidebar-day-of-webinar-700x500-1.jpg)
![[Advertisement]](https://ipwatchdog.com/wp-content/uploads/2026/02/Anaqua-Feb-12-2026-sidebar-700x500-1.jpg)
![[Advertisement]](https://ipwatchdog.com/wp-content/uploads/2026/02/Ankar-AI-Feb-17-2025-sidebar-700x500-1.jpg)
![[Advertisement]](https://ipwatchdog.com/wp-content/uploads/2025/12/LIVE-2026-sidebar-regular-price-700x500-1.jpg)







![[Advertisement]](https://ipwatchdog.com/wp-content/uploads/2021/12/WEBINAR-336-x-280-px.png)
![[Advertisement]](https://ipwatchdog.com/wp-content/uploads/2021/12/Ad-4-The-Invent-Patent-System™.png)







Join the Discussion
One comment so far.
Anon
August 4, 2025 11:50 amAbout time.