“The defendants sold bottles of Gilead-branded medicine with ‘spurious marks’—made-up pedigrees and other manipulations to the labeling, sealing, and bottle caps—that concealed the fact that the bottles were acquired from street-level buy back operations.” – Judge Ann Donnelly
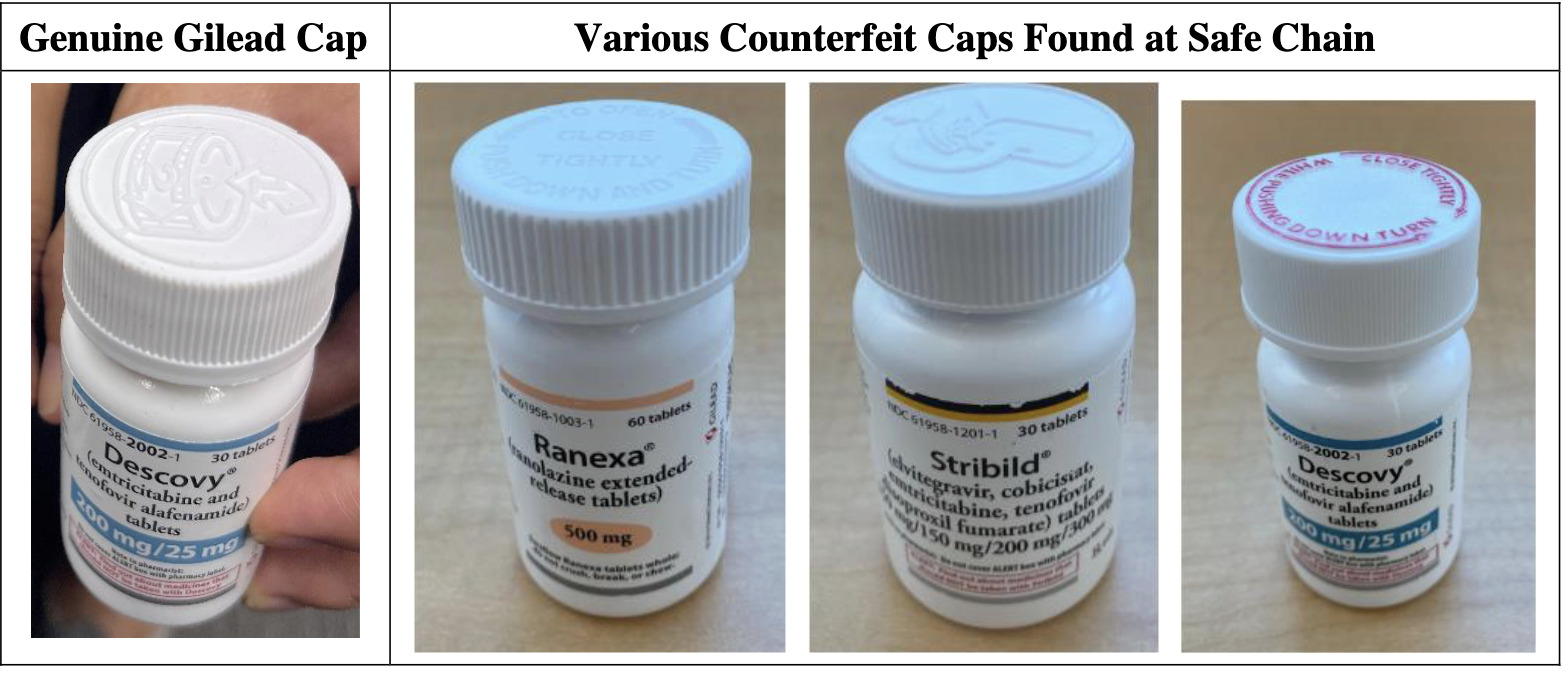 The U.S. District Court for the Eastern District of New York, in a decision published Monday, denied the defendants’ motions to vacate asset freezes in a case brought by Gilead alleging a massive HIV drug counterfeiting ring that involves “hundreds of millions of dollars’ worth” of fake medications.
The U.S. District Court for the Eastern District of New York, in a decision published Monday, denied the defendants’ motions to vacate asset freezes in a case brought by Gilead alleging a massive HIV drug counterfeiting ring that involves “hundreds of millions of dollars’ worth” of fake medications.
In January 2022, the court unsealed documents in the suit against a slew of defendants who Gilead said sold, marketed, and distributed counterfeits of its HIV medications. Gilead’s complaint sought immediate monetary and injunctive relief, including seizure at certain of the defendants’ premises, as well as relief for trademark and trade dress infringement and trademark dilution, among other alleged violations.
Gilead warned in an August, 2021, press release that it had become aware of counterfeit HIV drugs and explained how to verify the authenticity of dispensed pills. “Distributors not authorized by Gilead to sell Gilead-branded medicine have sold these counterfeits to pharmacies where genuine Gilead bottles have been tampered with a counterfeit foil induction seal or label and contain incorrect tablets,” said the release.
Judge: Gilead Met Its Burden
Today’s decision relates to the defendants Peter Khaim and Scripts, who filed motions for modification or dissolution of the asset freezes against them. In the court’s decision denying the motions and entering them as preliminary injunctions, Judge Ann Donnelly said that, while Scripts had the right to challenge the temporary restraining order initially entered by the court, Gilead had met the burden of making the requisite showing for entry of a preliminary injunction.
Gilead alleged that every bottle of the 54,000 Scripts sold has a counterfeit “pedigree,” which is the “record purporting to show the chain of all sales or transfers of that drug,” according to a Gilead press release. Scripts attempted to argue that the Drug Supply Chain Security Act (DSCSA) pre-empts Gilead’s Lanham Act claims “because that statute gives the FDA the ‘exclusive authority to decide claims involving suspect pedigrees.’” But the court disagreed, holding that “[l]itigation over the alleged fabrication of the chains of sale does not, as Scripts suggests, ‘neutraliz[e] the choice’ that the DSCA provides it and other industry actors. Nor has the FDA intervened to ask the Court to dismiss the pedigree-related claims.”
Scripts also argued that since the fake pedigrees were attached to genuine Gilead medications, they were actually “gray market goods” rather than counterfeits and not covered by the Lanham Act. But the district court judge agreed with Gilead that “the pedigrees render the bottles materially different from genuine bottles of Gilead medication, deceiving customers and ‘subvert[ing its] quality control efforts.’” Judge Donnelly continued:
“The defendants sold bottles of Gilead-branded medicine with ‘spurious marks’—made-up pedigrees and other manipulations to the labeling, sealing, and bottle caps—that concealed the fact that the bottles were acquired from street-level buy back operations. This is sufficient to state a claim that the alleged unauthorized use of the Gilead-branded bottles created ‘a likelihood of confusion regarding’ the source of the medications.”
Khaim did not contest the asset freeze but sought to modify it so it was restricted to only certain properties named. The court denied this motion “without prejudice to renewal, if they can show, ‘through documentary proof, that particular assets are not proceeds of counterfeiting activities so as to warrant exemption’ of those assets as consistent with this order.”
Anticounterfeiting Awareness Resolution
Also on Monday, Senators Chris Coons (D-DE) and Chuck Grassley (R-IA) issued a press release about their introduction of a resolution recognizing the month of August as “National Anti-Counterfeiting and Consumer Education and Awareness Month.” The Senate passed the resolution on July 27.
“Counterfeits can put consumers in danger, harm the intellectual property of inventors and creators, and hurt our economy,” Coons said in the press release. “The more we can do to raise attention to the harms of counterfeit goods, the stronger American innovation will be.”

![[IPWatchdog Logo]](https://ipwatchdog.com/wp-content/themes/IPWatchdog%20-%202023/assets/images/temp/logo-small@2x.png)

![[Advertisement]](https://ipwatchdog.com/wp-content/uploads/2024/04/UnitedLex-May-2-2024-sidebar-700x500-1.jpg)
![[Advertisement]](https://ipwatchdog.com/wp-content/uploads/2024/04/Artificial-Intelligence-2024-REPLAY-sidebar-700x500-corrected.jpg)
![[Advertisement]](https://ipwatchdog.com/wp-content/uploads/2024/04/Patent-Litigation-Masters-2024-sidebar-700x500-1.jpg)

![[Advertisement]](https://ipwatchdog.com/wp-content/uploads/2021/12/WEBINAR-336-x-280-px.png)
![[Advertisement]](https://ipwatchdog.com/wp-content/uploads/2021/12/2021-Patent-Practice-on-Demand-recorded-Feb-2021-336-x-280.jpg)
![[Advertisement]](https://ipwatchdog.com/wp-content/uploads/2021/12/Ad-4-The-Invent-Patent-System™.png)






Join the Discussion
No comments yet.