“Because Arbutus chose to incorporate several references into both the prior art patent and ’127 patent, that material became incorporated into the host document.” – CAFC
 The U.S. Court of Appeals for the Federal Circuit (CAFC) today affirmed in a precedential decision the Patent Trial and Appeal Board’s (PTAB’s) finding that Moderna Therapeutics proved certain claims of Arbutus Biopharma Corporation’s mRNA delivery patent invalid as anticipated.
The U.S. Court of Appeals for the Federal Circuit (CAFC) today affirmed in a precedential decision the Patent Trial and Appeal Board’s (PTAB’s) finding that Moderna Therapeutics proved certain claims of Arbutus Biopharma Corporation’s mRNA delivery patent invalid as anticipated.
U.S. Patent No. 9,404,127 is titled “Non-liposomal Systems for Nucleic Acid Delivery” and is directed to “an invention that provides stable nucleic acid-lipid particles (‘SNALP’) that have a non-lamellar structure and ‘comprise a nucleic acid . . . methods of making the SNALP, and methods of delivering and/or administering the SNALP.’” SNALP has a three-dimensional structure that is either a lamellar morphology or non-lamellar (pictured).
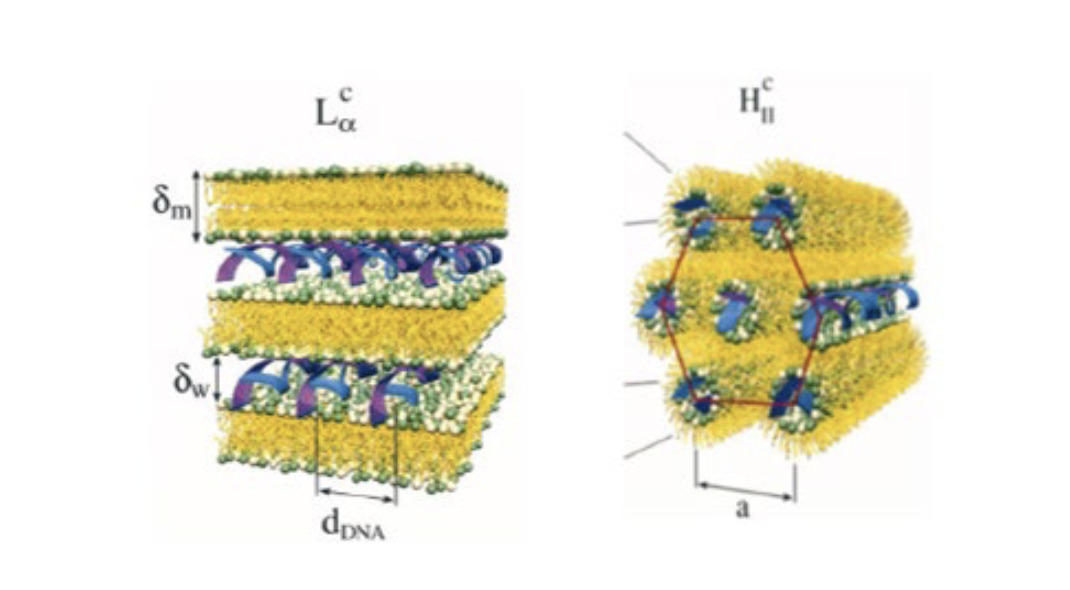
The main issue before the PTAB was “whether claim 1(d) of the ’127 patent—wherein at least about 95% of the particles in the plurality of particles have a non-lamellar morphology (the “Morphology Limitation”)—is inherently disclosed” in Arbutus’ earlier-issued U.S. Patent No. 8,058,069, which Moderna argued anticipated every challenged claim of the ‘127 patent. Moderna specifically argued “that the Morphology Limitation, while not expressly mentioned in the prior art, is an ‘inherent natural property’ resulting from the lipid composition of the formulation and formation process.”
Addressing whether the Morphology Limitation is met or inherently anticipated, the CAFC first said that the ‘127 and ‘069 patent disclosures disclose “the same formulations with ‘almost identical wording’” and thus the Board’s determination that the formulations are “the same or essentially the same” across both patents was supported by substantial evidence. Next, the Direct Dilution Method (DDM) process is disclosed and described in the same way across both patents, said the CAFC. And finally, since the formulations and processes were found to be similarly disclosed in both patents, the court considered whether the PTAB’s finding that making the disclosed formulations using the disclosed process naturally resulted in a composition having the Morphology Limitation was proper.
Turning to whether dependent claims 3 and 8–12 of the ’127 patent are anticipated, the court noted that dependent claim 3 recites “[t]he composition of claim 1, wherein the nucleic acid is mRNA” and that “the Board found that the ’069 patent explicitly discloses that the nucleic acid can be mRNA.” Claim 8 recites “[t]he composition of claim 1, wherein the nucleic acid is fully encapsulated in the particles” and the “Board found that the prior art patent explicitly discloses that the nucleic acid may be fully encapsulated within the lipid portion of the particle.” Thus, the finding that claims 3 and 8 were anticipated is supported by substantial evidence, said the CAFC. As to claim 9, the court agreed with the Board’s finding that “the structures recited in claim 9 were inherent properties of the non-lamellar Morphology Limitation that is, in turn, inherently anticipated in claim 1.”
With respect to claims 10-12, which recite percentage ranges for a lipid component of claim 1, the CAFC disagreed with Arbutus’ argument that “using different references to arrive at the limitations of the claimed ranges is error because the Board did not evaluate the claims as a whole.” The court explained: “When a patent claims a chemical composition in terms of ranges and a single prior art reference discloses a composition that falls within each of the ranges, the range is anticipated.”
Moderna had cited to several disclosures in the prior art patent, through incorporation by reference, to find the disclosure of cationic lipid amounts, and, upon review, the PTAB found that the prior art patent and its incorporated references disclose each of the claimed ranges.
The CAFC said substantial evidence supported the Board’s findings, explaining:
“Because Arbutus chose to incorporate several references into both the prior art patent and ’127 patent, that material became incorporated into the host document. Those disclosures, when reviewed as a whole, sufficiently disclose and describe claims 10–12, rendering each anticipated.”
Moderna began attempts to invalidate three of Arbutus’ LNP delivery patents at the PTAB in 2018. The inter partes reviews (IPRs) had mixed results, with one win for Moderna on the ‘127 patent, one partial win (U.S. Patent No. 9,364,435) and one loss (the ‘069 patent).
Image Source: Deposit Photos
Image ID:480067362
Copyright:niphon

![[IPWatchdog Logo]](https://ipwatchdog.com/wp-content/themes/IPWatchdog%20-%202023/assets/images/temp/logo-small@2x.png)

![[Advertisement]](https://ipwatchdog.com/wp-content/uploads/2024/04/UnitedLex-May-2-2024-sidebar-700x500-1.jpg)
![[Advertisement]](https://ipwatchdog.com/wp-content/uploads/2024/04/Artificial-Intelligence-2024-REPLAY-sidebar-700x500-corrected.jpg)
![[Advertisement]](https://ipwatchdog.com/wp-content/uploads/2024/04/Patent-Litigation-Masters-2024-sidebar-700x500-1.jpg)

![[Advertisement]](https://ipwatchdog.com/wp-content/uploads/2021/12/WEBINAR-336-x-280-px.png)
![[Advertisement]](https://ipwatchdog.com/wp-content/uploads/2021/12/2021-Patent-Practice-on-Demand-recorded-Feb-2021-336-x-280.jpg)
![[Advertisement]](https://ipwatchdog.com/wp-content/uploads/2021/12/Ad-4-The-Invent-Patent-System™.png)






Join the Discussion
No comments yet.