“When it comes to clinical trials related to cancer alone, the United States has registered 43,244 of the 90,168 total registered clinical trials, compared to the second highest country, France, with 8,579.”
 The U.S. Chamber of Commerce released a report today predicting that proposals by the Biden Administration to impose price controls on certain pharmaceuticals will reduce the number of clinical trials “by thousands across all categories of research examined and by up to 75% in some therapeutic areas,” eventually turning the United States into a “research desert.”
The U.S. Chamber of Commerce released a report today predicting that proposals by the Biden Administration to impose price controls on certain pharmaceuticals will reduce the number of clinical trials “by thousands across all categories of research examined and by up to 75% in some therapeutic areas,” eventually turning the United States into a “research desert.”
The report comes on the heels of a Federal Register Notice last week that proposed a framework for expanding the use of march-in rights under the Bayh-Dole Act to circumstances in which qualifying drugs are priced too high.
Today’s report, however, focuses mostly on the Inflation Reduction Act (IRA) provisions that allow the Department of Health & Human Services (HHS) to set prices for certain drugs under Medicare. While the IRA frames the pricing scheme as a “negotiation,” those who oppose it have said that the penalty for refusing to accept a negotiated price—up to 1900% of daily drug sales—essentially gives companies no choice.
A Chamber press release said that the report estimates the following results of the IRA price control mechanism:
- Cancer research drops 60%, including for promising, next-generation biologics
- Heart disease research declines 12%, the leading cause of death in the U.S.
- Obesity trials diminish by 70%, despite affecting over 40% of Americans
- Up to 75% cut in research funds from all privately funded clinical trials
These estimates were achieved by conducting a comparison between the United States and other developed, major Organization for Economic Cooperation and Development (OECD) economies that have imposed price controls on the biopharmaceutical sector. Ultimately, the report found, such policies “cripple the free market and enterprise and, as this report demonstrates, cripple life saving innovation.”
According to the report, the IRA’s price control mechanism is even stricter than some other countries’ price management systems. For example, in France and Germany, products that can demonstrate an improved clinical benefit are awarded price premiums, whereas the IRA “focuses almost exclusively on expenditure reduction regardless of a given product’s therapeutic value,” said the report.
In many OECD economies where strict price controls are currently employed, there is evidence that “many new products and medical innovations never make it into the market.” Looking at cancer medicines, for instance, recent data on the launch of new medicines in this category show that “even Germany and the United Kingdom, which in the past have been the closest, are falling behind the United States,” with only 56% of 104 products launched globally since 2017 launching in Germany, France, Italy or the United Kingdom compared to the 80% launched in the United States. Furthermore, patients in economies with strict price controls generally have longer waits for new medicines—in France and Spain, between 5 and 600 days.
As for clinical trials and R&D, the United States was compared with Australia, Canada, France, Germany, Italy, Japan, South Korea, Spain, and the United Kingdom, using the two major international clinical trials registries: The World Health Organization’s International Clinical Trials Registry Platform (ICTRP) and the U.S. National Institutes of Health’s clinicaltrials.gov. According to the report, the United States has hosted nearly the same number of clinical trials overall (48% of the total) as Australia, Canada, France, Germany, Italy, Japan, South Korea, Spain, and the United Kingdom combined (52%).
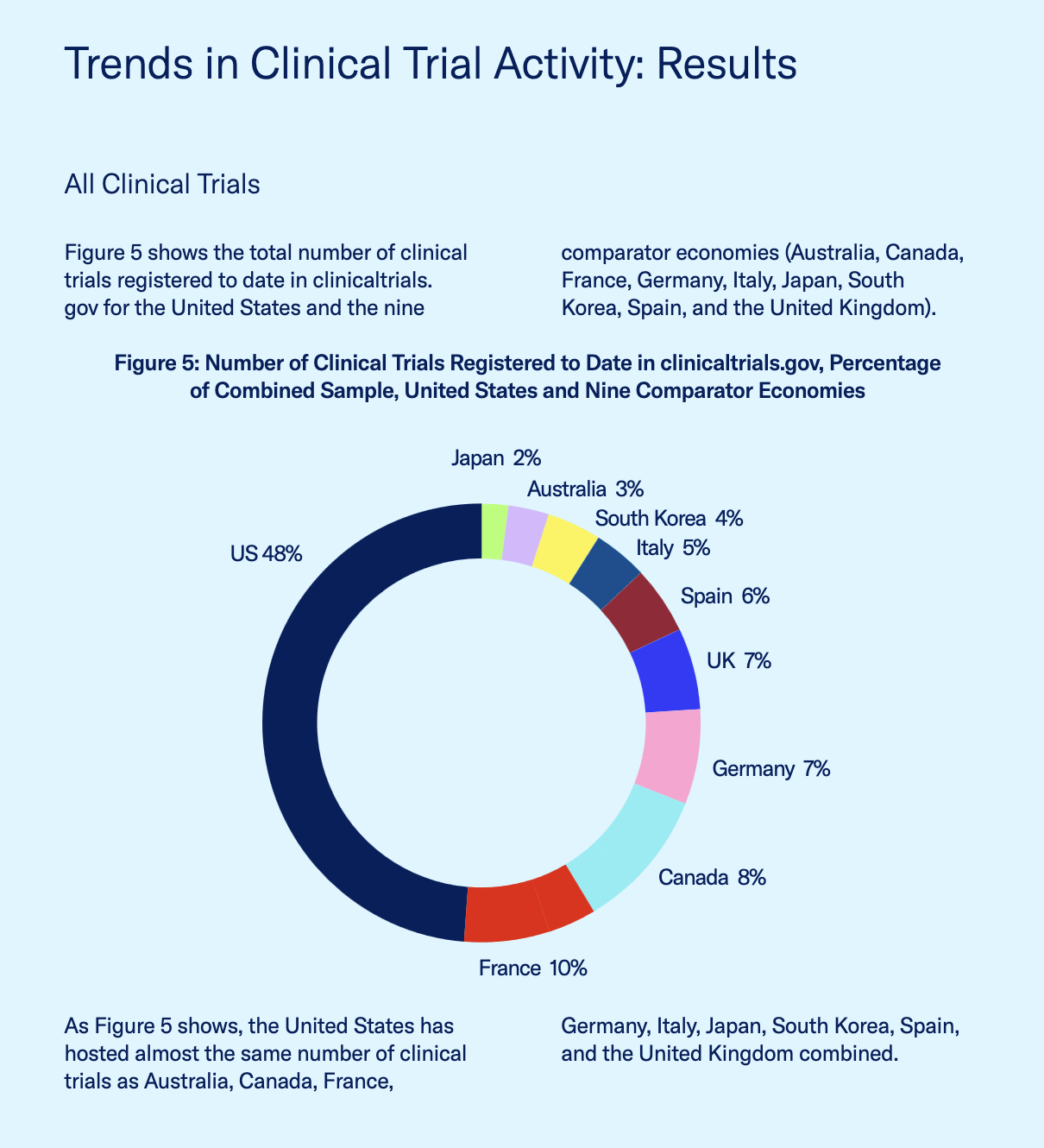
Source: U.S. Chamber Report
When it comes to clinical trials related to cancer alone, the United States has registered 43,244 of the 90,168 total registered clinical trials, compared to the second highest country, France, with 8,579. For Alzheimer’s disease, the United States hosted 1,625 of the 3,317 total registered clinical trials, or 49%.
And when it comes to investment in these trials, the report showed that industry accounts for a large percentage. The report looked at data for industry sponsorship of clinical trials for biologics, cancer research and Alzheimer’s disease and found that 34% to 43% of all phases of clinical trials in these areas was sponsored by industry. Those numbers increase when isolated for early-phase research.
The report also includes with models of the potential negative impact of the IRA price controls “by comparing levels of clinical research standardized for population size in the United States and the average for the nine comparator OECD economies.” The report estimates, for example, that the number of clinical trials in the area of obesity would be reduced by nearly 69% overall and 75.84% for early-phase research; in oncology by 37% overall and 54.13% for early phase; and for diabetes the reduction would be 37.49% overall and 49.11% for early phase research.
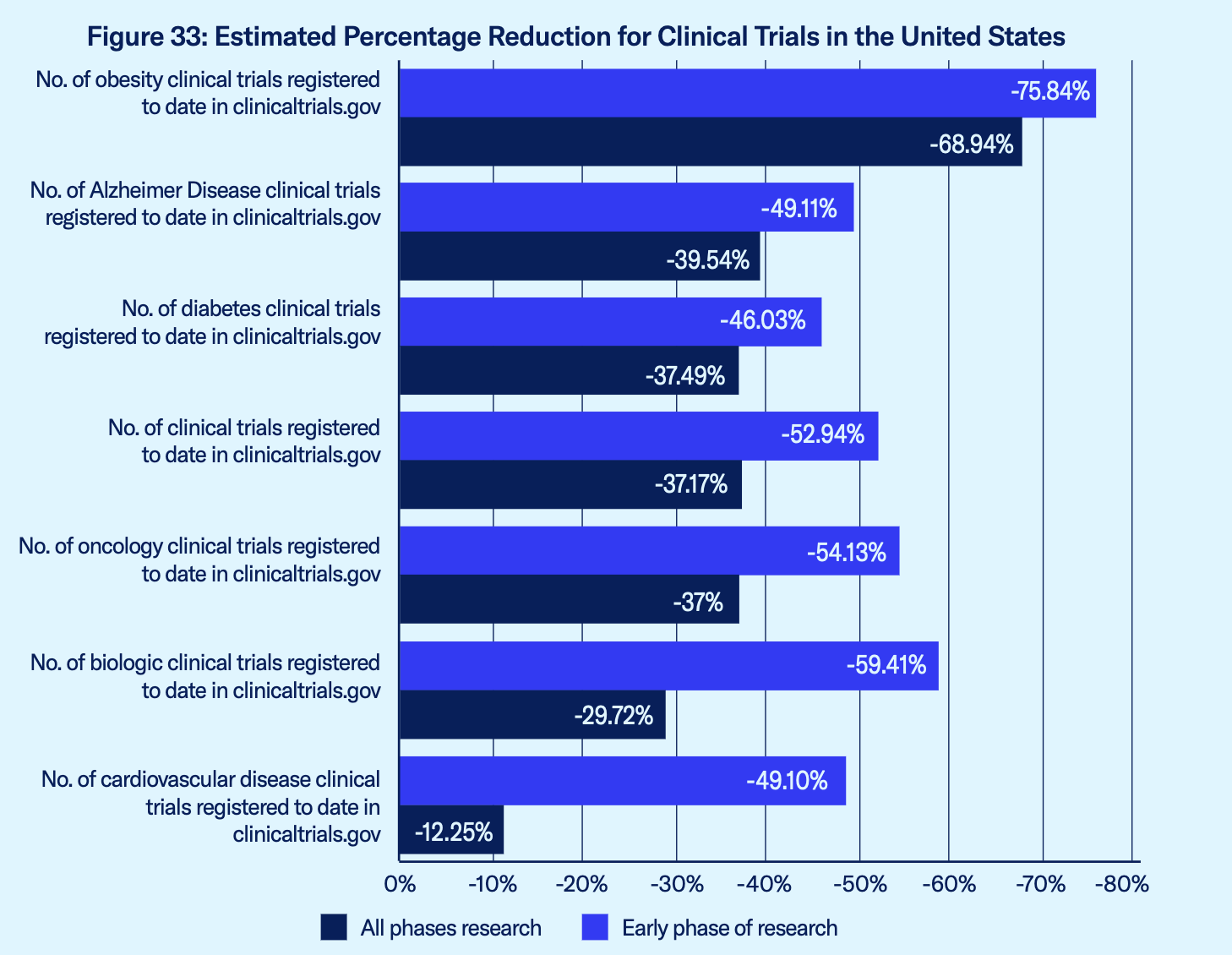
Source: U.S. Chamber Report
Ultimately, the report claims, the United States will go from world leadership in biopharmaceutical innovation to “the emergence of research deserts”:
“With fewer resources, it stands to reason that life sciences manufacturers will have less to invest in R&D and will be less likely to develop new life sciences products and services at the same rate as in the past. This logic holds true whether a new medicine was developed by a public or private research entity….
All health systems struggle with rising costs; this is not a uniquely American phenomenon. But the solution is not to impose a “take it or leave it” system of price controls targeting medicines that will, inevitably, undermine the ability of our life sciences innovation ecosystem to continue to function at such a high level.”
“The evidence is clear, the administration’s pursuit of price controls will reduce funding for research and clinical trials, resulting in fewer therapies for diseases like cancer, Alzheimer’s, and heart disease,” said the Chamber’s Executive Vice President, Tom Quaadman.
He added: “By taking away the dollars needed for private sector research, cures and treatments may never happen, creating a clear and present danger for scientific progress and the healthy well-being of all Americans.”
Image Source: Deposit Photos
Author: viendomundo
Image ID: 634949086

![[IPWatchdog Logo]](https://ipwatchdog.com/wp-content/themes/IPWatchdog%20-%202023/assets/images/temp/logo-small@2x.png)

![[Advertisement]](https://ipwatchdog.com/wp-content/uploads/2024/04/UnitedLex-May-2-2024-sidebar-700x500-1.jpg)
![[Advertisement]](https://ipwatchdog.com/wp-content/uploads/2024/04/Artificial-Intelligence-2024-REPLAY-sidebar-700x500-corrected.jpg)
![[Advertisement]](https://ipwatchdog.com/wp-content/uploads/2024/04/Patent-Litigation-Masters-2024-sidebar-700x500-1.jpg)

![[Advertisement]](https://ipwatchdog.com/wp-content/uploads/2021/12/WEBINAR-336-x-280-px.png)
![[Advertisement]](https://ipwatchdog.com/wp-content/uploads/2021/12/2021-Patent-Practice-on-Demand-recorded-Feb-2021-336-x-280.jpg)
![[Advertisement]](https://ipwatchdog.com/wp-content/uploads/2021/12/Ad-4-The-Invent-Patent-System™.png)






Join the Discussion
One comment so far.
Mercedes Meyer
December 13, 2023 06:57 amIn considering your statistics, are the percentage of clinical trials by nation necessarily representative for each of the European nations when compared to the US without also considering each nation’s population and their GDP? I think the number of new drugs per country are more representative. Interestingly, India is the world maker of vaccines, but there I think you would have to look at efficacy as well.