“This after-the-fact manufactured genus was filed by [Gilead] as a competitive play against [Atea], which is in advanced clinical trials with bemnifosbuvir to treat HCV and COVID19…” – Atea Pharmaceuticals’ PGR petition
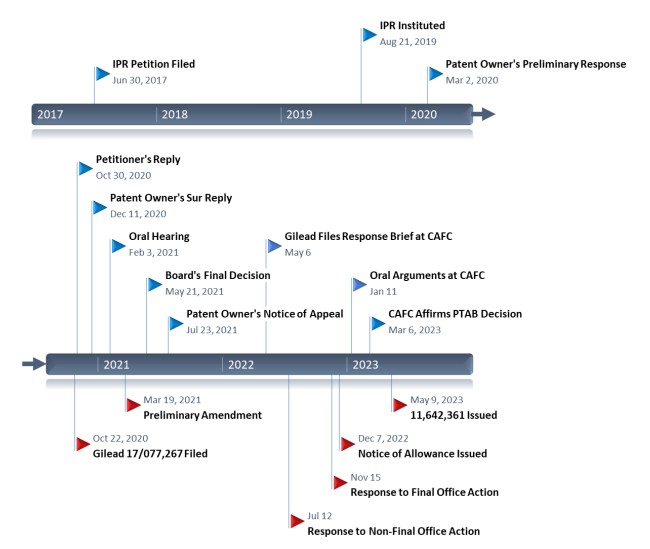
 This past week, IPWatchdog was made aware of a petition for post-grant review (PGR) proceedings at the Patent Trial and Appeal Board (PTAB) that raises interesting arguments about allegedly inconsistent statements on patentability made during legal proceedings by pharmaceutical firm, Gilead Sciences. Filed in early August, the PGR petition from Atea Pharmaceuticals cites 35 U.S.C. § 112 arguments previously raised by Gilead in other cases to challenge the validity of Gilead’s own patent claims that were allegedly obtained to block Atea’s competing hepatitis C virus (HCV) treatments.
This past week, IPWatchdog was made aware of a petition for post-grant review (PGR) proceedings at the Patent Trial and Appeal Board (PTAB) that raises interesting arguments about allegedly inconsistent statements on patentability made during legal proceedings by pharmaceutical firm, Gilead Sciences. Filed in early August, the PGR petition from Atea Pharmaceuticals cites 35 U.S.C. § 112 arguments previously raised by Gilead in other cases to challenge the validity of Gilead’s own patent claims that were allegedly obtained to block Atea’s competing hepatitis C virus (HCV) treatments.
Gilead’s Specification Contains Inadequate ‘Blaze Marks’ to Support Claimed Subgenus
At issue in Atea Pharmaceuticals’ PGR petition is Gilead Sciences’ U.S. Patent No. 11642361, Nucleoside Phosphoramidate Prodrugs. Issued this May, the ‘361 patent claims the use of a compound derived from nucleoside phosphoramidates as an inhibitor of ribonucleic acid (RNA) replication processes for treating HCV infection in patients. Atea’s PGR petition alleges that, of the more than 15 billion compounds contained within the genus claim of the ‘361 patent, only one compound has its synthesis disclosed by the patent’s specification. “This after-the-fact manufactured genus was filed by [Gilead] as a competitive play against [Atea], which is in advanced clinical trials with bemnifosbuvir to treat HCV and COVID19 and will compete with Gilead’s Sovaldi and Veklury products,” Atea’s PGR petition reads.
In racing to obtain these patent rights, Gilead allegedly took patentability positions conflicting with its own invalidity arguments in other PTAB proceedings. This March, the U.S. Court of Appeals for the Federal Circuit issued a precedential decision affirming the PTAB’s invalidation of University of Minnesota patent rights challenged in inter partes review (IPR) proceedings petitioned by Gilead. In that case, which also involved patent claims covering nucleosides with antiviral qualities, the Federal Circuit agreed with the PTAB that the university’s patent and priority applications failed to disclose adequate “blaze marks” within the specification to support the claimed subgenus of compounds. The absence of blaze marks in parent applications prevented the University of Minnesota from claiming a priority date earlier than Gilead’s anticipating patent application.
An expert declaration submitted by Atea in support of its PGR petition similarly contends that the specification to Gilead’s ‘361 patent, which claims priority to the same parent application asserted as prior art against the University of Minnesota, contains no blaze marks directing a person of ordinary skill in the art to the specific subgenus covered by claim 1. The disclosure of a single compound cannot provide adequate written description support for a genus including 15 billion compounds under Gilead’s own arguments in the University of Minnesota case, Atea contends. Atea’s PGR petition also provides a timeline of filings allegedly showing Gilead’s inconsistent arguments on Section 112 patentability during an overlapping period of time.
Failure to Disclose Inconsistent Legal Positions Allegedly Violates Duty of Candor
Gilead’s prosecution of the ‘361 patent was also contemporaneous with an opposition proceeding filed by Gilead at the European Patent Office (EPO) to challenge a European patent issued to UK biopharmaceutical company NuCana. NuCana’s patent claimed phosphoramidate derivatives of nucleotides that are allegedly similar in structure and chemical makeup to the genus claimed in Gilead’s ‘361 patent. During the opposition proceedings, Gilead argued that certain nucleotides within NuCana’s genus claim were impossible to make or too unstable for pharmaceutical applications. Gilead’s own expert declaration in those proceedings contended that skilled artisans would face “a research project” in creating claimed unsynthesized compounds with the desired functionality.
The amount of experimentation required to create new substituent variations of the one compound disclosed in Gilead’s ‘361 patent is made more difficult by the fluorination chemistry techniques involved, Atea’s petition asserts. The organic chemistry literature review required to identify proper synthesis techniques is substantial by itself, and Gilead’s own expert declaration in the NuCana opposition claimed that it would take anywhere from six months to three years for a skilled chemist to synthesize each claimed compound. Further, Atea cites Gilead’s expert testimony from UK High Court proceedings against NuCana indicating that certain substituent variations in nucleotide or nucleoside analogues, like those within the genus claim of Gilead’s ‘361 patent, could negatively impact the compound’s antiviral activity.
Atea’s PGR petition alleges that Gilead’s ‘361 patent should not be able to claim priority to parent applications filed before Atea’s own patent application covering bemnifosbuvir, which was published by the USPTO more than six years before Gilead presented current claim 1 of the ‘361 patent to the USPTO. Atea also argues that Gilead’s failure to disclose its patentability submissions from the NuCana proceedings during prosecution of the ‘361 patent violated the company’s duty of candor to the USPTO.
Image Source: Deposit Photos
Author: iqoncept
Image ID: 59574913

![[IPWatchdog Logo]](https://ipwatchdog.com/wp-content/themes/IPWatchdog%20-%202023/assets/images/temp/logo-small@2x.png)

![[Advertisement]](https://ipwatchdog.com/wp-content/uploads/2024/04/UnitedLex-May-2-2024-sidebar-700x500-1.jpg)
![[Advertisement]](https://ipwatchdog.com/wp-content/uploads/2024/04/Artificial-Intelligence-2024-REPLAY-sidebar-700x500-corrected.jpg)
![[Advertisement]](https://ipwatchdog.com/wp-content/uploads/2024/04/Patent-Litigation-Masters-2024-sidebar-700x500-1.jpg)

![[Advertisement]](https://ipwatchdog.com/wp-content/uploads/2021/12/WEBINAR-336-x-280-px.png)
![[Advertisement]](https://ipwatchdog.com/wp-content/uploads/2021/12/2021-Patent-Practice-on-Demand-recorded-Feb-2021-336-x-280.jpg)
![[Advertisement]](https://ipwatchdog.com/wp-content/uploads/2021/12/Ad-4-The-Invent-Patent-System™.png)






Join the Discussion
One comment so far.
Pro Say
August 30, 2023 02:34 pmGee. Inconsistent arguments.
Like the inconsistent Section 101 Big Tech arguments between their challenges to the patents / claims of others . . . and the arguments they make in order to obtain their own patents / claims.
In tightly-related and overlapping technologies.
OTHER’S computer / Internet / software claims are ineligible . . . while OURS are not.
Patent Offices, District Courts, and the CAFC, kindly take note of their hypocrisy.