“In Japan, method-of-treatment claims should be rewritten as purpose-limited composition claims to allow the applicant substantially the same scope of patent right as the original claims.”
In the United States, claims directed to methods of treating/diagnosing human disease are patentable. On the other hand, in Japan, such claims are unpatentable. Therefore, the applicant is required to rewrite or delete the claims when a patent application (e.g., Patent Cooperation Treaty application) containing such claims enters the Japanese national phase and is examined.
In this article, I offer my personal views on how to rewrite method-of-treatment claims for Japanese examination. I will particularly focus on claims that may or may not conform to Japanese patent practice while past Japanese patent cases and the current patent system are taken into account.
How to Rewrite Method-of-Treatment Claims for the JPO
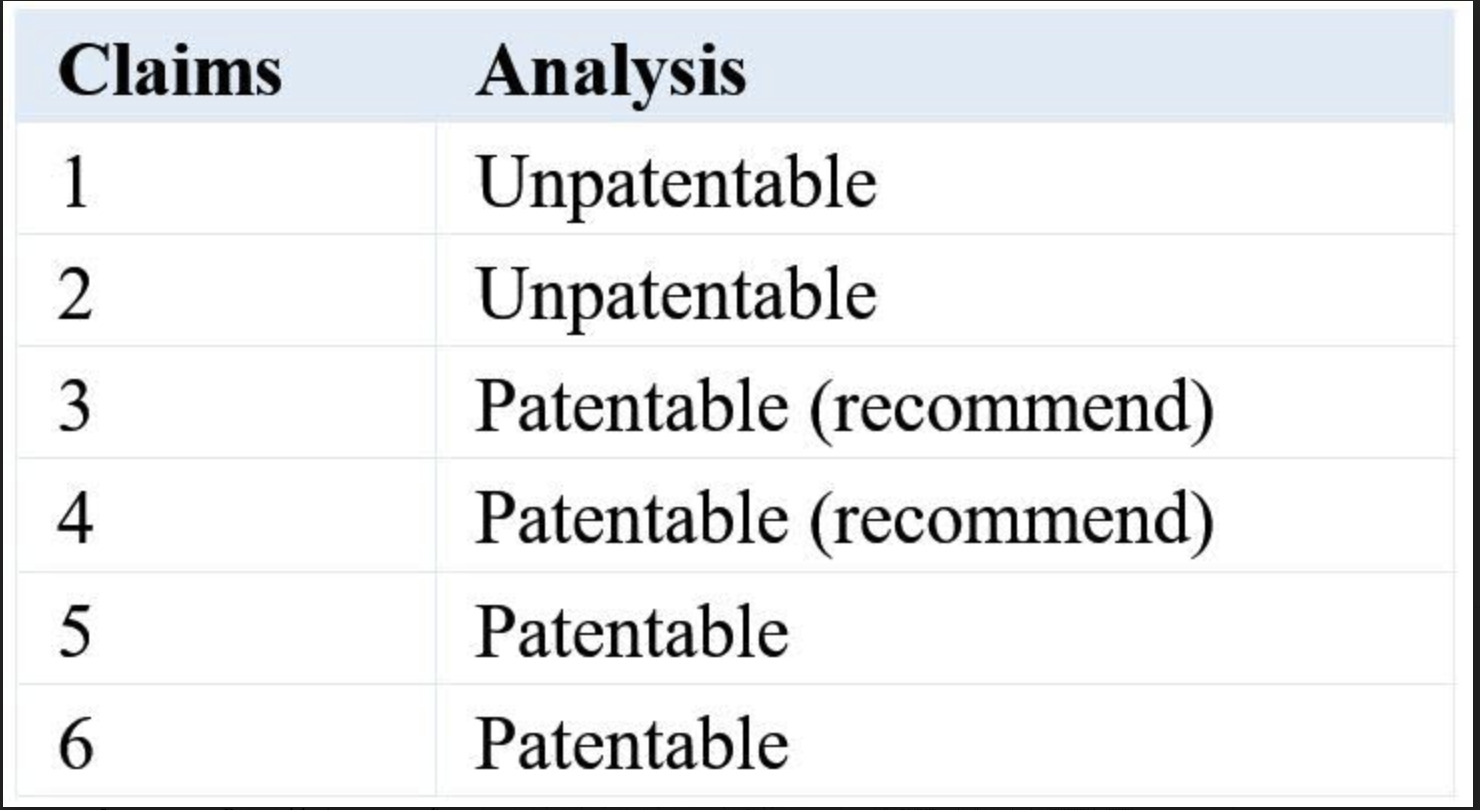
The following shows examples of typical claims involving methods of treatment or pharmaceutical purpose, as well as the results of analyzing their patentability in Japan. Since the only language accepted by the Japanese Patent Office (JPO) is Japanese, the corresponding Japanese claims are posted on this website (JPR) for your reference.
——————–
- A method of treating a malignant tumor comprising administering to a patient in need thereof a compound X.
- Use of a compound X for the treatment of a malignant tumor.
- A pharmaceutical composition for the treatment of a malignant tumor, comprising a compound X.
- A pharmaceutical composition for use in the treatment of a malignant tumor, comprising a compound X.
- Use of a compound X in the manufacture of a medicament for the treatment of a malignant tumor.
- A compound X for use in the treatment of a malignant tumor.
Claim 1 is a method-of-treatment claim. In Japan, this claim is not allowed because it does not meet the requirement of industrial applicability (Section 29(1), main paragraph of the Japanese Patent Law). Accordingly, the applicant must amend the claim. During examination in Japan, claim 2 is likewise construed as a method-of-treatment claim. The U.S. patent cases in this form include US 9,126,941 (XTANDI patent).
Claim 3 or 4 is a purpose-limited composition claim for pharmaceutical use. This type of claim can meet the requirement of industrial applicability, and has a broad and clear scope. Therefore, it is recommended that the applicant should include claim 3 or 4 in the application. It is unnecessary to include both claims 3 and 4. Either one is sufficient to cover a typical pharmaceutical use or medical use. Here, it is also possible to set forth a “therapeutic agent” or “drug” instead of “pharmaceutical composition.” The Japanese patent cases in this form include JP 4,178,032 (ABILIFY tablets patent) and JP 5,138,753 (Xtandi tablets patent).
Claim 5 is a Swiss-type claim. This type of claim can meet the requirement of industrial applicability. However, claim 5 is usually not recommended because it is unclear whether the claim falls under a production method or a simple method (other than production methods). In Japan, the production method covers a product (including an imported product). However, the simple method does not cover it. Thus, which method the claim falls under is an important issue to consider. If claim 3 or 4 is listed in the claims, there is no problem with listing an additional claim 5.
Claim 6 is a purpose-limited compound claim for pharmaceutical use (claims 4 and 6 can be classified as so-called EPC 2000-style claims). Claim 6 can meet the requirement of industrial applicability. In this type of claim, the pharmaceutical use is directed to the compound and, therefore, JPO examiners are supposed to ignore the limitation of therapeutic use in the examination (i.e., the compound in claim 6 usually lacks novelty over known compound X). Therefore, claim 6 is not recommended.
In view of the above, when a patent application containing a method-of-treatment claim like claim 1 enters the national phase in Japan, the claim should be rewritten (amended) into a purpose-limited composition claim such as claim 3 or 4. The timing of claim amendment, generally speaking, is at the time of request for examination (due three years from the PCT filing date) or at the time of national entry (due 30 months from the priority date).
Meanwhile, claims involving combination therapy, dose and dosage or patients can also be set forth as purpose-limited composition claims. These claims are explained below.
How to Write Combination Therapy Claims
Following are examples of combination therapy claims that are allowed in the United States but not in Japan. The U.S. patent cases in this form include US 8,952,018 TAFINLAR and MEKINIST combination patent) and US 9,084,776 (OPDIVO and YERVOY combination patent).
——————–
- A method of treating a malignant tumor comprising administering to a patient in need thereof a compound X and a compound Y.
- A method of treating a malignant tumor comprising administering to a patient in need thereof a compound X, wherein the patient has been administered a compound Y prior to the administration of the compound X.
——————–
The following are examples of combination therapy claims that are usually allowed in Japan.
——————–
- A pharmaceutical composition for the treatment of a malignant tumor comprising a compound X, wherein the pharmaceutical composition is administered in combination with a compound Y.
- A pharmaceutical composition for the treatment of a malignant tumor comprising a compound X, for use in combination treatment with a compound X and a compound Y.
- A pharmaceutical composition for the treatment of a malignant tumor comprising a compound X, wherein the treatment comprises administering a compound X and a compound Y in combination.
- A pharmaceutical composition for the treatment of a malignant tumor comprising a compound X, wherein a patient to be treated in the treatment is administered compound Y prior to, simultaneously with, or after administration of the compound X.
- A pharmaceutical composition for the treatment of a malignant tumor comprising a compound Y, wherein the pharmaceutical composition is administered in combination with a compound X.
- A pharmaceutical composition for the treatment of a malignant tumor comprising compound Y, for use in combination treatment with a compound X and a compound Y.
- A pharmaceutical composition for the treatment of a malignant tumor comprising a compound Y, wherein the treatment comprises administering a compound X and a compound Y in combination.
- A pharmaceutical composition for the treatment of a malignant tumor comprising a compound Y, wherein a patient to be treated in the treatment is administered compound X prior to, simultaneously with, or after administration of the compound Y.
- A pharmaceutical composition for the treatment of a malignant tumor comprising a compound X or a compound Y, wherein the pharmaceutical composition is used in combination treatment with a compound X and a compound Y.
- A pharmaceutical composition for the treatment of a malignant tumor, comprising a compound X and a compound Y.
——————–
Claims 9 to 18 are purpose-limited composition claims for combination therapy. Claims 9-12 differ from claims 13-16 in the scope of patent right because the former composition comprises a compound X and the latter composition comprises a compound Y. Thus, for a typical claim format, it is recommended that any one of Claims 9-12 and any one of Claims 13-16 are included. Although not a typical style, the claims could be combined into one claim, as in claim 17. Claim 18 covers a combination tablet and combination capsule. It is recommended that claim 18 also be listed as a dependent or independent claim, just in case.
The Japanese patent cases in the form of claim 9 include JP 5,872,377 (patent on the combination of OPDIVO i.v. Infusion and YERVOY Injection) and JP 6,563,554 (patent on the combination of KYMRIAH for i.v. infusion and ACTEMRA for i.v. infusion). The Japanese patent cases in the form of claim 17 include JP 6,408,993 (Obinutuzumab and Venetoclax combination patent).
How to Write Dose and Dosage Claims
Below are examples of claims that involve pharmaceutical purpose characterized by dose and dosage that are usually allowed in Japan.
——————–
- A pharmaceutical composition for use in the treatment of a malignant tumor comprising a compound X, wherein the treatment comprises administering 30 to 40 ?g/kg body weight of a compound A to a patient once every two weeks.
- A pharmaceutical composition for use in the treatment of a malignant tumor comprising a compound X, wherein 30 to 40 ?g/kg body weight of a compound X is administered to a patient once every two weeks.
——————–
Claims 19 and 20 are purpose-limited composition claims for pharmaceutical use characterized by dose and dosage. Either one is considered sufficient to cover the invention on typical dose and dosage. The Japanese patent cases in the form of claim 20 include JP 6,869,400 (patent on IMBRUVICA Capsules) and JP 6,143,664 (patent on RITUXAN Intravenous Infusion).
It is relatively difficult to obtain a patent for dose and dosage from the viewpoint of novelty and inventive step. However, once granted, the patent is effective for life cycle management of a pharmaceutical product. In particular, in Japan, the patent terms of multiple patents (e.g., a substance, purpose, dose and dosage) for a single product can be extended (maximum extension of five years for each patent). This can increase the value of such a claim (i.e., it may prevent the entry of generic drugs for a long period of time after the expiration of patent term of the substance patent).
How to Write Patient Classification Claims
Here are examples of claims that involve pharmaceutical purpose characterized by patient classification that are usually allowed in Japan.
——————–
- A pharmaceutical composition for use in the treatment of a malignant tumor in a patient comprising a compound X, wherein the patient has a protein Z of 10 ng/mL or greater in plasma.
- A pharmaceutical composition for use in a method of treatment of a malignant tumor comprising a compound X, wherein the method of treatment comprises administering to a patient a compound X, and the patient has a protein Z of 10 ng/mL or greater in plasma.
——————–
Claims 21 and 22 are purpose-limited composition claims for pharmaceutical use characterized by patient classification. The either one is considered sufficient to cover the invention characterized by typical patient classification. The Japanese patent cases in the form of claim 21 include JP 6,351,828 (patent on Cyramza Intravenous Injection) and JP 6,473,845 (patent on Entyvio for I.V. Infusion).
Choose the Right Rewrite
Method-of-treatment claims are patentable in the United States, but are not necessarily allowed in other countries. For example, in Japan, Europe and China, method-of-treatment claims are unpatentable. Therefore, it is common practice for European applicants to rewrite the claims to purpose-limited product claims and for Chinese applicants to rewrite them as Swiss-type claims. In Japan, as described above, the claims should be changed to purpose-limited composition claims. This allows the applicant to retain substantially the same scope of patent right as the original method-of-treatment claims.

![[IPWatchdog Logo]](https://ipwatchdog.com/wp-content/themes/IPWatchdog%20-%202023/assets/images/temp/logo-small@2x.png)


![[Advertisement]](https://ipwatchdog.com/wp-content/uploads/2024/05/LexisNexis-May-16-2024-sidebar-700x500-1.jpg)
![[Advertisement]](https://ipwatchdog.com/wp-content/uploads/2024/04/Patent-Litigation-Masters-2024-sidebar-last-chance-700x500-1.jpg)
![[Advertisement]](https://ipwatchdog.com/wp-content/uploads/2024/05/Patent-Portfolio-Management-2024-sidebar-super-early-bird-with-button-700x500-1.jpg)
![[Advertisement]](https://ipwatchdog.com/wp-content/uploads/2024/05/Artificial-Intelligence-2024-Getting-AI-Patents-Allowed-sidebar-700x500-1.jpeg)

![[Advertisement]](https://ipwatchdog.com/wp-content/uploads/2021/12/WEBINAR-336-x-280-px.png)
![[Advertisement]](https://ipwatchdog.com/wp-content/uploads/2021/12/2021-Patent-Practice-on-Demand-recorded-Feb-2021-336-x-280.jpg)
![[Advertisement]](https://ipwatchdog.com/wp-content/uploads/2021/12/Ad-4-The-Invent-Patent-System™.png)







Join the Discussion
No comments yet.