“In re Baird illustrates that the disclosure of a genus in the prior art is not necessarily a disclosure of every species that is a member of that genus. Patentees can use this concept to their advantage in applying for solid-form patents.”
 Pharmaceutical products are typically dosed as solids, liquids (e.g., solutions) or gases. In gases and liquids, the molecules are tumbling; in solids, however, the molecules are essentially frozen in place, forming three-dimensional networks. When those networks are ordered, they are called crystalline. A crystalline solid can be thought of as containing a “unit cell” which is propagated in three dimensions – i.e., a crystal. The solid-state properties of a drug can often greatly affect drug delivery, such as how much and how fast an active ingredient can be delivered from ingestion by a patient to the site of action (Shekunov, B.Yu & York, Peter. (2000). Crystallization Processes in Pharmaceutical Technology and Drug Delivery Design. Journal of Crystal Growth. 211. 122-136. 10.1016/S0022-0248(99)00819-2).
Pharmaceutical products are typically dosed as solids, liquids (e.g., solutions) or gases. In gases and liquids, the molecules are tumbling; in solids, however, the molecules are essentially frozen in place, forming three-dimensional networks. When those networks are ordered, they are called crystalline. A crystalline solid can be thought of as containing a “unit cell” which is propagated in three dimensions – i.e., a crystal. The solid-state properties of a drug can often greatly affect drug delivery, such as how much and how fast an active ingredient can be delivered from ingestion by a patient to the site of action (Shekunov, B.Yu & York, Peter. (2000). Crystallization Processes in Pharmaceutical Technology and Drug Delivery Design. Journal of Crystal Growth. 211. 122-136. 10.1016/S0022-0248(99)00819-2).
Sometimes, the same chemical compound can exist in different crystalline forms depending on how the compound is crystallized. Until about 30 years ago, for example, carbon was known to have two crystalline forms – graphite and diamond. Although both are carbon, they each have vastly different properties. Diamond is the hardest known substance and is used in many applications such as industrial drills and fine jewelry. Graphite, on the other hand, is a lubricant and as a marking source in pencils.
Organic molecules can also exist in different crystalline forms. Cocoa butter, a prime ingredient of chocolate, exists in six different crystalline forms. The crystalline form found in most chocolates, Form V, imparts a smooth sensation when consumed, but it is not the most stable crystalline form. That feature goes to Form VI, which renders chocolate dull and soft and is not pleasant to taste – leaving a sandy sensation on the tongue. For this reason, the confectionary industry spends considerable sums to make sure that Form V is maintained within chocolate (Roth, K. (2010). Chocolate – The Noblest Polymorphism II. ChemViews).
This behavior where the same chemical compound can exist in multiple crystalline forms is called polymorphism, with each crystalline form called a polymorph. When the compound is an element, as with carbon, it is referred to as allotropism. Predicting whether a chemical compound will be polymorphic, what that polymorph might be, and the properties thereof are notoriously challenging feats. Because polymorphs or other crystalline forms can have significantly different drug solubility and dissolution properties, it is not surprising that they are often the subject of patent applications in the pharmaceutical arts.
Solid Forms – Wakix® Example
Pitolisant hydrochloride, marketed as Wakix®, was approved by the United States Food and Drug Administration (FDA) in August of 2019 for the treatment of narcolepsy. The patentees hold U.S. Patent No. 7,169,928 (the ‘928 patent), covering the composition of matter directed to the pitolisant molecule and “its pharmaceutically acceptable salts, hydrates, hydrated salts, or its optical isomers, racemates, diastereoisomers or enantiomers” (Schwartz, Jean-Charles et al. 2007. US 7,169,928 B2. Col. 160 1. 66 col. 161 1. 3). The ‘928 patent is based on a Patent Cooperation Treaty (PCT) application filed July 29, 1999. Upon ultimate grant as a U.S. Patent, it was awarded a 188-day patent term adjustment resulting in an expiration date of February 2, 2020. Thus, the composition of matter expired shortly after approval and long before the end of regulatory exclusivity. A second patent, however, was granted, U.S. Patent No. 8,207,197 (the ‘197 patent), which is also directed to pitolisant. Claim 1 of the ‘197 patent is provided below:
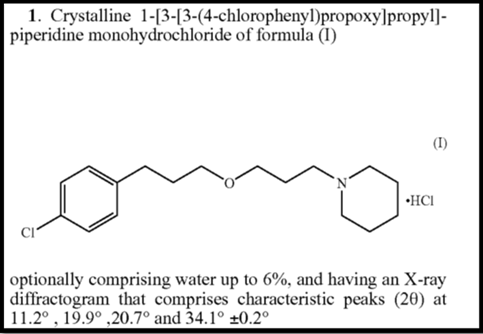
Note that claim 1 is narrower than claim 13 of the original ‘928 patent covering the free base of the API and “its pharmaceutically acceptable salts.” Claim 1 of the ‘197 patent is limited to the hydrochloride salt, optionally water, and contains additional limitations directed to four peaks in an x-ray powder diffractogram (XRPD) of the API.
The ‘197 patent was filed as a PCT on February 6, 2006. The corresponding U.S. application matured into a patent with a generous 1,115-day patent-term adjustment to provide an expiration date of February 25, 2029, more than nine years after the expiration date of the original ‘928 patent. Both the ‘928 and ‘197 patents are drug substance patents and are (or were in the case of the ‘928 patent) listed in the Orange Book. Further, on June 10, 2021, the Federal Register reported that a request for a 389-day patent term extension was made by the patentees (86 FR 30947 Fed. Cir. 2021). If finalized, this would result in an expiration date of March 21, 2030, an impressive 10 years beyond the original composition of matter patent.
Shown below is the chemical structure found in the Wakix® label:
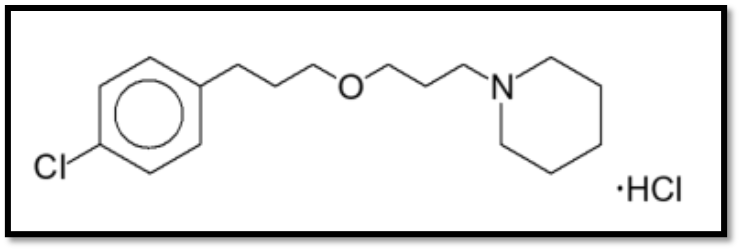
Claim 13 of the ‘928 patent presumably reads on this chemical structure. The compound, an HCl salt, is a pharmaceutically acceptable salt of pitolisant. With respect to claim 1 of the ‘197 patent, if we assume that the active ingredient sold under the name Wakix® has the four XRPD peaks claimed and has a water content that “optionally” goes up to 6%, then claim 13 would in fact be a picture claim of the Wakix® API covering little else. Thus, the Wakix® example provides at least two patents, each of which cover the marketed API, but with different scope. It is this difference which enables a solid-form patent strategy.
Genus/Species Framework
The ability to get multiple drug substance patents covering the same compound rests on how patent law views scope of claims and in particular, the genus-species relationship. In re Baird concerns a patentee that attempted to claim an invention directed to a flash fusible toner comprising a polyester of bisphenol A and an aliphatic dicarboxylic acid (In re Baird, 16 F.3d 380, 381, 382, 383 (Fed. Cir. 1994)). After the application was rejected, the Applicant appealed, asserting that the patent application’s rejection for obviousness was incorrect. The contested claim 1 reads as follows:
- A flash fusible toner comprising a binder resin which is a bisphenol A polyester containing an aliphatic di[carboxylic] acid selected from the group consisting of succinic acid, glutaric acid and adipic acid.
The board at the USPTO rejected this claim as obvious, considering U.S. Patent No. 4,634,649 (the ‘649 patent), which related to composition of “the polymeric esterification product of a dicarboxylic acid and a diphenol of the following generic formula.”
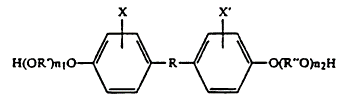
The ‘649 patent, in its descriptions of this general structure and of the claimed dicarboxylic acids, listed broad ranges of the substituents R, R’, R’’, X’, and X’’ (16 F.3d 381, 382 Fed. Cir. 1994). The broad claims of the ‘649 patent clearly included the structure of the Baird compound, as shown below, and USPTO rejected the Baird application on these grounds as being obvious.
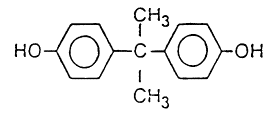
The Applicant argued that there was nothing in the broad claims of the ‘649 patent to motivate or make obvious to a POSA “to select bisphenol A from the vast number of diphenols covered by the generic formula” (16 F.3d. 383 Fed. Cir. 1994). The court agreed with the Applicant, stating that “the fact that a claimed compound may be encompassed by a disclosed generic formula does not by itself render that compound obvious.” Specifically, the court rejected the notion that such a broad claim of chemical structures renders all encompassed compounds obvious, where the broader claims did not disclose, suggest, or state as preferable the narrower claim (16 F.3d. 382, 383 Fed. Cir. 1994).
In re Baird illustrates that the disclosure of a genus in the prior art is not necessarily a disclosure of every species that is a member of that genus. Patentees can use this concept to their advantage in applying for solid-form patents, wherein the prior art compound becomes the genus and the solid form the species. Given this relationship, it is important to understand that under typical circumstances when a genus is small, the genus may render the species within it obvious if the species can be “envisaged” (Application of Petering, 301 F.2d 676, 133 U.S.P.Q. 275 (C.C.P.A. 1962)). Of course, with respect to solid forms, the level of unpredictability is typically higher than with synthetic chemistry since the structure of a crystalline compound cannot readily be discerned from merely looking at a chemical structure.
This discussion will be familiar to the practicing pharmaceutical patent attorney in the guise of selection inventions. Selection inventions occur when a later filed patent, which could be in the same family or not as the earlier patent, claims a species, whereas the earlier patent claims a genus. This very same approach can be used with solid forms wherein the earlier composition of matter claims, which directed to a chemical species act in fact as a genus wherein the later filed solid form patent claims a narrower “sub species” limited by the solid-state features of the claim. It is this genus-species relationship which permits the patentability of later-filed solid form patents. In our next article, we will discuss other aspects of patentability relevant to solid forms.
For more on this topic, check out IPWatchdog’s March 1 webinar, “Pharmaceutical Salts and other Complexes – A Solid Form Strategy,” sponsored By Barash Law, which can be viewed here.

![[IPWatchdog Logo]](https://ipwatchdog.com/wp-content/themes/IPWatchdog%20-%202023/assets/images/temp/logo-small@2x.png)

![[Advertisement]](https://ipwatchdog.com/wp-content/uploads/2024/03/IP-Copilot-Apr-16-2024-sidebar-700x500-scaled-1.jpeg)
![[Advertisement]](https://ipwatchdog.com/wp-content/uploads/2024/04/Patent-Litigation-Masters-2024-sidebar-early-bird-ends-Apr-21-last-chance-700x500-1.jpg)

![[Advertisement]](https://ipwatchdog.com/wp-content/uploads/2021/12/WEBINAR-336-x-280-px.png)
![[Advertisement]](https://ipwatchdog.com/wp-content/uploads/2021/12/2021-Patent-Practice-on-Demand-recorded-Feb-2021-336-x-280.jpg)
![[Advertisement]](https://ipwatchdog.com/wp-content/uploads/2021/12/Ad-4-The-Invent-Patent-System™.png)






Join the Discussion
3 comments so far.
Bob Kelson
March 10, 2022 05:59 pmA very good summary. Clear. I will keep this article as a reference.
B
March 9, 2022 01:41 pmThank you, Me. Barash
I found this article informative and helpful having dealt with a similar issue last year
C. Whewell
March 9, 2022 01:31 pmGreat stuff, nice writeup, I love pharmachem ! It ties in also with KSR’s operative phrase of “obvious to try.” When the genus is small (brake lever configs.), the POSITA can presumably envisage all of the species. If we are talking about the stereo isomers of C-4 hydroxy carboxylates, one can probably draw them all out on a single sheet of paper. But what if we are considering C-22 hydroxy carboxylates? Whoa, I need a lot more paper ! And then there is the step of selecting the particular species from the large genus….. what motivation exists to propel a POSITA to a particular one ? It will need to be more than a mere change in an art-recognized, results-effective variable !!
Nice article, thanks.