“Through demanding from drug companies either greater royalties or certain price concessions in order to avoid a lawsuit, we may see the federal government turn to patents as another tool to address drug pricing.”
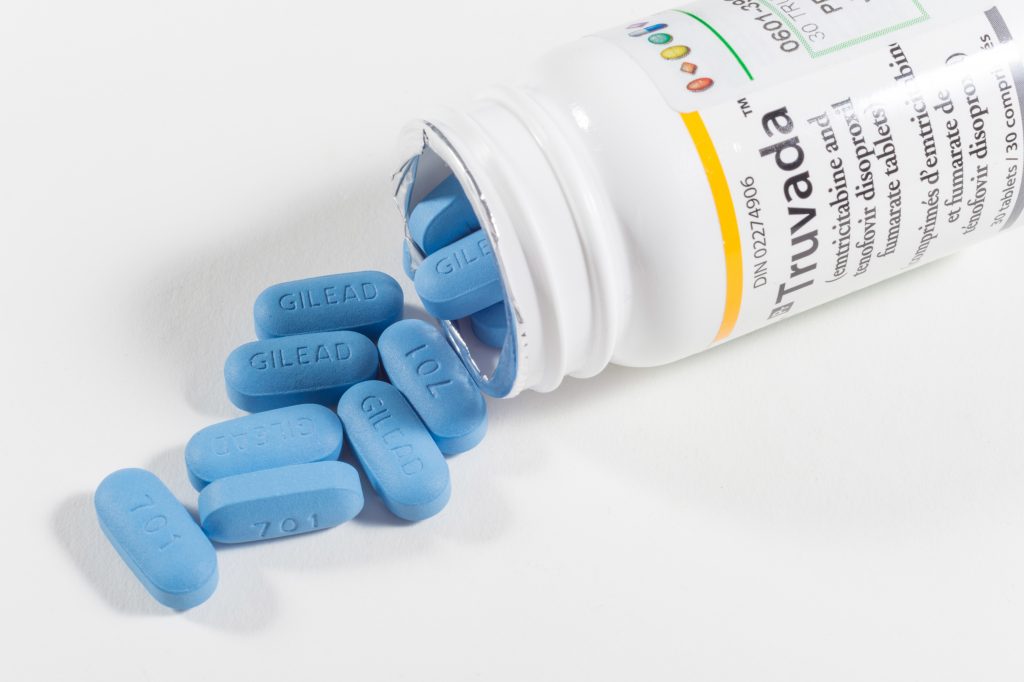 In most respects, the patent complaint recently filed against Gilead is perfectly ordinary. It was filed in the U.S. District Court for the District of Delaware, the most common venue for patent infringement lawsuits, and alleged that a pharmaceutical company’s drug sales infringed the plaintiff’s patents on uses of the sold drug. The defendant’s response has so far been similarly unexceptional: Gilead has filed petitions for Inter Partes Review (IPR) of each asserted patent (see links below), arguing that those patents are invalid over the prior art, either as obvious or as entirely anticipated. What is remarkable about this widely reported lawsuit, however, is that the plaintiff is the government of the United States. Complaint, United States v. Gilead, C.A. No. 19-2103-MN (D. Del. Nov. 6, 2019) (“Complaint”).
In most respects, the patent complaint recently filed against Gilead is perfectly ordinary. It was filed in the U.S. District Court for the District of Delaware, the most common venue for patent infringement lawsuits, and alleged that a pharmaceutical company’s drug sales infringed the plaintiff’s patents on uses of the sold drug. The defendant’s response has so far been similarly unexceptional: Gilead has filed petitions for Inter Partes Review (IPR) of each asserted patent (see links below), arguing that those patents are invalid over the prior art, either as obvious or as entirely anticipated. What is remarkable about this widely reported lawsuit, however, is that the plaintiff is the government of the United States. Complaint, United States v. Gilead, C.A. No. 19-2103-MN (D. Del. Nov. 6, 2019) (“Complaint”).
IPR2019-01453-1
IPR2019-01454-1
IPR2019-01455-1
IPR2019-01456-1
A Strategic Shift?
The federal government does not commonly sue over patents on drugs or medical technology. It appears that the Department of Health and Human Services (HHS) has asserted fewer than 10 such patents in litigation over the last two decades. The historically low rate of lawsuits is unsurprising – the government’s goal is to bring new technologies onto the market in order to improve the health of the American public, whereas patents are fundamentally a method of excluding technology from the market. The lawsuit against Gilead may indicate a significant change in the government’s patent enforcement strategy.
Typically, when collaborating with government agencies such as the National Institutes of Health (NIH) or the Centers for Disease Control and Prevention (CDC), drug companies agree to license any resulting patents obtained by the government. Gilead has allegedly refused to do so, Complaint ¶ 9, asserting instead that the patents are invalid. Although the government’s lawsuit can be viewed simply as an attempt to enforce patents where an alleged infringer has refused to pay for a license, the rarity of such lawsuits by the government indicates that other factors may be at play. For example, it’s possible that one of the driving forces may have been public concern over the prices of the accused products, sold under the brand names Truvada® and Descovy®. Some language in the Complaint supports this interpretation. See id. ¶¶ 178, 186-88, 193-95. Moreover, some supporters of the lawsuit argue that it provides the government with leverage to demand that Gilead make its medicines more affordable or potentially donate more products to those in need.
The price of Truvada® has, perhaps, been the subject of particularly fervent activism. Nevertheless, Truvada® is hardly unique among pharmaceuticals in being expensive. The Trump administration and HHS Secretary Alex Azar have repeatedly stated their commitment to decreasing the price of drugs across the board. As such, it is possible that this lawsuit marks the beginning of a new approach by the federal government to reducing drug prices. Through demanding from drug companies either greater royalties (perhaps to fund medical programs or government procurement of drugs) or certain price concessions in order to avoid a lawsuit, we may see the federal government turn to patents as another tool to address drug pricing. Drug companies may need to be wary of patent enforcement suits that would have seemed very unlikely a year ago.
Potential Consequences
A possible consequence of such a strategy, and perhaps even of this single lawsuit, is a deterioration of the relationship – and, potentially, a reduction in collaboration – between HHS and the pharmaceutical industry. It is unlikely that any drug company could totally insulate itself from government research (for example, one study concluded that basic research funded by NIH contributed in some way to every new drug approved from 2010 to 2016). Nevertheless, pharmaceutical companies’ desire to avoid a patent lawsuit may lead to caution that hinders the creation of new treatments. Drug developers may, for example, become reluctant to expand upon (potentially patentable) research by government scientists. They may also refuse to cooperate with research efforts involving their products, such as by donating drug samples (as Gilead did), if they suspect that any resulting patents will be enforced against them.
At present, it is difficult to tell whether the Gilead suit is a sea change or an illusion of one. Potentially, however, it represents a change in government patent enforcement strategy that could have significant consequences for patients and industry. Patent practitioners and pharmaceutical companies should watch this suit carefully and stay attentive to future enforcement trends.

![[IPWatchdog Logo]](https://ipwatchdog.com/wp-content/themes/IPWatchdog%20-%202023/assets/images/temp/logo-small@2x.png)


![[Advertisement]](https://ipwatchdog.com/wp-content/uploads/2024/04/Patent-Litigation-Masters-2024-sidebar-early-bird-ends-Apr-21-last-chance-700x500-1.jpg)

![[Advertisement]](https://ipwatchdog.com/wp-content/uploads/2021/12/WEBINAR-336-x-280-px.png)
![[Advertisement]](https://ipwatchdog.com/wp-content/uploads/2021/12/2021-Patent-Practice-on-Demand-recorded-Feb-2021-336-x-280.jpg)
![[Advertisement]](https://ipwatchdog.com/wp-content/uploads/2021/12/Ad-4-The-Invent-Patent-System™.png)







Join the Discussion
4 comments so far.
angry dude
November 27, 2019 12:27 amHIGH TREASON
Joachim Martillo
November 26, 2019 10:38 amCan the US government assert sovereign immunity to USPTO review? Such review does not really make sense.
TFCFM
November 26, 2019 10:28 amCL&JK: “Through demanding from drug companies either greater royalties (perhaps to fund medical programs or government procurement of drugs) or certain price concessions in order to avoid a lawsuit, we may see the federal government turn to patents as another tool to address drug pricing.”
Although greater license royalties for government-patented-inventions and lower drug prices in general are both admirable (if vague) goals, I fail to see how this lawsuit furthers either goal in an appropriate manner.
As background for anyone who hasn’t been paying attention to this dispute, there’s no dispute that Gilead made the drug for treatment before the government claims to have invented its additional use for prevention. The IPRs and the refusal of Gilead to license are both based on allegations that it was obvious to use the treatment-drug for prevention purposes. Also, the complaint doesn’t assert that Gilead directly infringes, but rather that Gilead induces others to infringe, because others directly infringe using Gilead’s drug.
Discovery and trial may reveal whether Gilead is, genuinely, taking active steps to induce others to use its drug for prevention purposes (facially, at least, this does not appear to be happening). If Gilead is genuinely acting badly, then the suit is justifiable to recover in this instance and to incent others not to deliberately infringe government patents. However, if the situation is instead as it appears outwardly (Gilead is not inducing others; others are just using Gilead’s drug for purposes publicized by the government and others), then this suit is more troubling.
If the government is pursuing this suit with little or no justification “just to pressure” Gilead into taking a license and/or into lowering its prices on unrelated drugs, then the suit is no different than assigning FBI/CIA/military agents to “shadow” drug company employees, than submitting the defendant to annual IRS audits without other justification, than ordering the FDA to ‘reevaluate’ every application or registration of the defendant, or than otherwise harassing a defendant that the government thinks can be strong-armed into conforming to a political official’s will. That the harassment is intended to achieve a good end (e.g., lower drug prices) does not make it justifiable in a civilized society.
It will be interesting to see what the facts turn out to be and to draw conclusions after we know them.
Pro Say
November 25, 2019 06:09 pmThe U.S. gov asserting patents? Wait; what?!
Just can’t wait for one or more courts to rule that the gov’s own patents are merely natural laws . . . are abstract . . . or both!
You know; do to the gov what they’re doing to all other inventors and patent owners.
You know; wiping out innovation left and right.
Hilarious!
p.s. So what do you think now, Congress? Time yet for that eligibility restoration you said was forthcoming?