 As the most recent election cycle in the United States, laws and voter measures legalizing the prescription and sale of medical marijuana had been passed in 28 states and the District of Columbia. November’s election saw medical marijuana measures pass in eight of the nine states where such legalization votes were taking place. This April, Forbes reported on a marijuana industry study which forecast the market for legal sales of marijuana in the United States would grow by 26 percent up to $7.1 billion during 2016.
As the most recent election cycle in the United States, laws and voter measures legalizing the prescription and sale of medical marijuana had been passed in 28 states and the District of Columbia. November’s election saw medical marijuana measures pass in eight of the nine states where such legalization votes were taking place. This April, Forbes reported on a marijuana industry study which forecast the market for legal sales of marijuana in the United States would grow by 26 percent up to $7.1 billion during 2016.
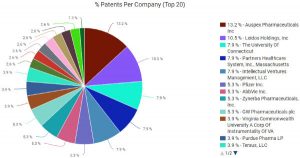 The patent space surrounding marijuana formulations for medical use is still wide open despite the current march towards legalization. According to patent portfolio analysis tools available through Innography, there are a total of 66 U.S. patent grants and 62 U.S. patent applications related to medical marijuana. As the pie chart here will show readers, the top three asset holders in this space are La Jolla, CA-based Auspex Pharmaceuticals (NASDAQ:ASPX), Leidos Holdings (NYSE:LDOS) and the University of Connecticut.
The patent space surrounding marijuana formulations for medical use is still wide open despite the current march towards legalization. According to patent portfolio analysis tools available through Innography, there are a total of 66 U.S. patent grants and 62 U.S. patent applications related to medical marijuana. As the pie chart here will show readers, the top three asset holders in this space are La Jolla, CA-based Auspex Pharmaceuticals (NASDAQ:ASPX), Leidos Holdings (NYSE:LDOS) and the University of Connecticut.
In the middle of November, New York City-based biotech firm AXIM Biotechnologies (OTCMKTS:AXIM) announced that it had secured a new patent grant claiming the use of all cannabinoids in controlled-release chewing gum products containing cannabinoids. U.S. Patent No. 9433601, titled Chewing Gum Compositions Comprising Cannabinoids, specifically claims a chewing gum composition containing 0.1 percent to 1 percent of cannabinoids provided within voids of a solid cellulose carrier, 25 percent to 85 percent of a gum base with a buffering agent, 10 percent to 35 percent of a sweetening agent and 1 percent to 10 percent of a flavoring agent; in this composition, cannabinoids begin release within three minutes of use and 30 percent of the cannabinoid composition is released within five minutes of use.
According to AXIM Biotech CEO George Anastassov, research and development for AXIM’s products goes back 14 years to a time where the political atmosphere surrounding medical marijuana was different. “The focus has been cannabinoid research from the very start,” Anastassov said, noting that his research focused on cannabinoids as an analgesic providing pain relief without the side effects of opioids or nonsteroidal anti-inflammatory drugs (NSAIDs). Anastassov and two partners began the Sanammad Foundation in the Netherlands before entering the American market through a 2012 business deal with Medical Marijuana, Inc. (OTCMKTS:MJNA) which gave Medical Marijuana a stake in Sanammad’s CanChew cannabinoid-containing chewing gum. CanChew Biotech was spun off as a private company in 2014 before it bought Nevada-based alternative energy developer AXIM International. Anastassov remarked that the AXIM purchase reflected what CanChew thought were synergistic interests given AXIM’s biofuel technologies and the biomass created by CanChew after cannabinoid extraction.
The recent issue of the ‘601 patent isn’t the only U.S. patent held by AXIM Biotech. The company also owns U.S. Patent No. 9023322, issued in May 2015 and given the same title as the ‘601 patent. The previous patent only claimed the use of cannabidiol (CBD) as a pharmaceutical agent, however, so the ‘601 patent expands the cannabinoids claimed by AXIM in its chewing gum products to include tetrahydrocannabinol (THC).
AXIM’s CanChew and MedChew RX cannabinoid chewing gum products are not the only pharmaceutical product being developed in AXIM’s product pipeline. The company is working on different cannabinoid delivery products including lozenges, toothpaste, mouthwash, eye drops and even topical applications like dermatological ointments and hand cream. According to Anastassov, AXIM has a particular interest in treating neurodegenerative diseases like multiple sclerosis (MS), Parkinson’s and Alzheimer’s diseases. “We know that all of these diseases are related to neuroinflammation,” Anastassov said, adding that certain cannabinoids have excellent anti-inflammatory characteristics. These cannabinoid treatments can also be useful against eczema and other diseases which are not neurodegenerative themselves but involve neurological processes.
Interestingly, there is growing evidence to support the idea that mastication, or the act of chewing, itself can show some effectiveness in slowing the process of neurodegenerative diseases. “We didn’t know about this when we started with chewing gums,” Anastassov said, although he added that it makes sense in retrospect as chewing nicotine gum is the most effective form of treating tobacco addiction. Anastassov noted that the act of chewing itself increases the velocity of the middle cerebral artery, induces the release of hormones like serotonin and dopamine, improves concentration and reduces stress.
AXIM Biotech’s products are specifically tailored for the medical marijuana industry; Anastassov said that the company is not targeting the recreational market at all. Even where smoking marijuana as a medical treatment is permissible by law, Anastassov suggests that there are significant health advantages to using a chewing gum treatment rather than inhaling smoke. AXIM’s products are designed to produce less 11-hydroxy-THC as a metabolic byproduct which the body creates when THC is ingested or inhaled. 11-hydroxy-THC is the metabolite responsible for the side effects of nausea and paranoia which are usually experienced by marijuana users. “We deliver a system which is just as efficient as smokable cannabis without negative effects,” Anastassov said. “We’re against any type of smokable material.”
Despite marijuana’s classification as a Schedule I drug, and therefore in the class of the most illegal drugs as defined by the Controlled Substances Act (CSA), AXIM Biotech has had little in the way of regulatory issues hampering either research and development or its process of obtaining a patent. Anastassov noted that the marijuana-based drug Marinol was approved by the U.S. Food and Drug Administration (FDA) back in the 1980s and has been on the market for more than 30 years. Perhaps more interesting is the fact that Anastassov said that the company holds 20 trademarks in various stages of issuance, none of which have been rejected because of their association with marijuana. In October, the U.S. Patent and Trademark Office’s Trademark Trial and Appeal Board (TTAB) rejected a trademark application filed by pre-loaded cannabis oil device maker JUJU Joints because the identified goods in the trademark applications were found to be paraphernalia for illegal drugs. AXIM Biotech might be focused on medical marijuana products, but Schedule I status means that a substance has no currently accepted medical use, so there does seem to be an inconsistency in how USPTO and TTAB interprets CSA.
The protection of AXIM Biotech’s intellectual property is very important to Anastassov. “We make sure that before we announce anything, we have IP protection,” he said. “People are good at reverse engineering other people’s ideas.” Despite holding certain developments under wraps, Anastassov noted that AXIM is very transparent and indicated that the company would have more to announce on new treatments “on the near horizon, not ten years from now.”
Although Anastassov has noted an “exuberant optimism” regarding the oncoming legalization of marijuana, he and the company have sought to keep themselves independent of the political atmosphere and he thinks that the enthusiasm has cooled down somewhat. He does add, however, that he thinks the political situation has helped AXIM Biotech and other companies in the medical marijuana space. Anastassov notes that the development of marijuana-based products across the industry is targeting an incredibly wide array of conditions including epilepsy, cancer, osteoporosis and infertility. “The potential of the plant is truly remarkable,” he said.

![[IPWatchdog Logo]](https://ipwatchdog.com/wp-content/themes/IPWatchdog%20-%202023/assets/images/temp/logo-small@2x.png)

![[Advertisement]](https://ipwatchdog.com/wp-content/uploads/2024/04/Patent-Litigation-Masters-2024-sidebar-early-bird-ends-Apr-21-last-chance-700x500-1.jpg)

![[Advertisement]](https://ipwatchdog.com/wp-content/uploads/2021/12/WEBINAR-336-x-280-px.png)
![[Advertisement]](https://ipwatchdog.com/wp-content/uploads/2021/12/2021-Patent-Practice-on-Demand-recorded-Feb-2021-336-x-280.jpg)
![[Advertisement]](https://ipwatchdog.com/wp-content/uploads/2021/12/Ad-4-The-Invent-Patent-System™.png)







Join the Discussion
2 comments so far.
Inventor Woes
January 9, 2017 04:08 pmFiling a patent for anything marijuana related seems like a huge waste of money. Nothing is gonna stop others from making their own weed chewing gum. Patents and the illicit drug market don’t mix very well I’m afraid. It’s not like other inventions, people will always find ways to get their product out.
Joachim CS Martillo
January 9, 2017 01:14 pmAs a parent I find a chewing gum delivery system and the following passage a little disturbing.
Yet I must note that when I was an undergraduate Joe’s Pizza of Cambridge, MA was reputed to use pizza pies as a delivery system for non-medical marijuana.